June 17, 2020
Development of Antibody-Oligonucleotide Complexes for Targeting Exosomal MicroRNA
Prof. Asako Yamayoshi (Department of Cell Biology, Institute of Biomedical Sciences) and her colleagues have developed novel antibody-oligonucleotide complexes for targeting exosomal mRNA with Prof. Hiroshi Sugiyama (Kyoto University), Prof. Hiroshi Harada (Kyoto University), Prof. Eishi Ashihara (Kyoto Pharmaceutical University) and Prof. Akira Murakami (Kyoto Institute of Technology).
MicroRNAs in exosomes (exosomal miRNAs) are considered as significant targets for cancer therapy. In general, anti-miR oligonucleotides, which have a sequence complementary to miRNA, are often used for the functional inhibition of miRNA; however, there is no useful strategy to regulate the function of miRNAs in exosomes in vivo, because exosomal miRNAs are encased in an exosome and do not have any uptake mechanisms for oligonucleotides, such as a cellular endocytosis pathway . Additionally, recent studies have revealed that integrin clusters on an exosome determine the organotropic uptake of the exosomes. If we want to introduce therapeutic nucleic acids (TNAs) to exosome-recipient cells before the exosome enters the cells in vivo, we have to identify the in vivo distribution profiles of the target exosome and have to develop functional oligonucleotides for specific delivery to the all kinds of recipient cells targeted by the exosome.
Prof. Asako Yamayoshi attempted to develop a novel drug delivery system using anti-exosome antibody–anti-miR oligonucleotide complexes (ExomiR-Tracker) to hijack exosomes to carry Anti-miR oligonucleotides inside exosome-recipient cells. They found that ExomiR-Tracker bound to the exosomes, and then, the complexes were introduced into the recipient cells. It was also found that Anti-miR oligonucleotides introduced into the recipient cells can exhibit inhibitory effects on exosomal-miRNA functions in vitro and in vivo.
Those results suggest that this strategy allows us to use exosomes as natural cargo for TNAs without exosome isolation. Exosomes are considered as potential cargo for the delivery of TNA, and there are several methods for the introduction of TNAs into isolate exosomes from plasma or cell cultures; however, despite the potential significance of exosomes, they continue to be challenging cargo mainly due to the lack of efficient exosome isolation technology and TNA-loading methods. This strategy would be a promising one for delivering various kinds of nucleic acid drugs to cancer cells.
This study has been published on line in Pharmaceutics on June 12, 2020.
https://www.mdpi.com/1999-4923/12/6/545
doi:10.3390/pharmaceutics12060545
Contact information
Asako Yamayoshi, Ph.D.
Professor
Chemistry of Functional Molecules
Graduate School of Biomedical Sciences
Nagasaki University
Phone: +81-95-819-2438
E-mail: asakoy*nagasaki-u.ac.jp (change * to @)
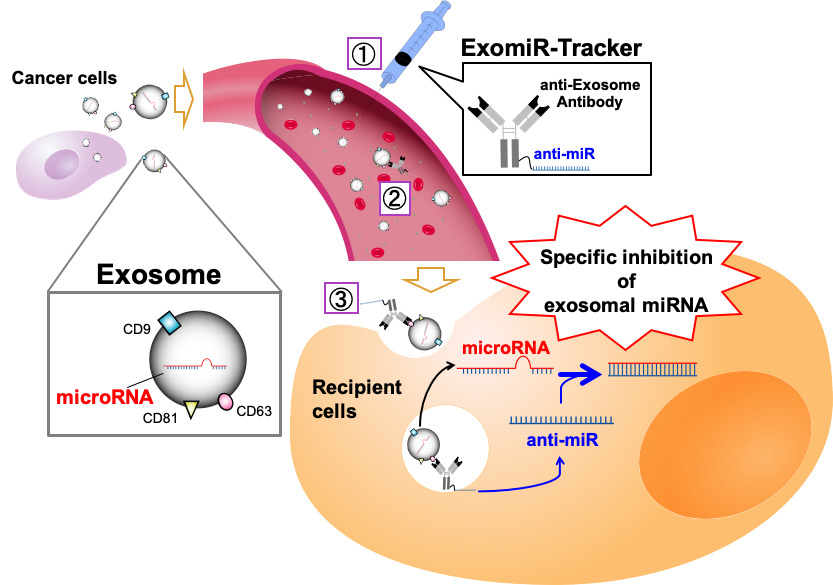
Figure 1 Schematic representation of the concept of this study
ExomiR-Tracker binds onto the surface of the exosome, leading to incorporation into the cells and the subsequent inhibition of exosomal miRNA functions.










