December 07, 2020
Discovery of a surface protein that regulates expression of genes encoding components of the type IX secretion system in a periodontal pathogen
Dr. Hideharu Yukitake, Department of Microbiology and Oral Infection, Graduate School of Biomedical Sciences, Nagasaki University, and his colleagues discovered a cell surface protein that regulates the gene expression of the proteins that make up the type IX secretion system (T9SS) of a major periodontal pathogen, Porphyromonas gingivalis, and revealed its structure by X-ray crystallography in a joint research with Professor Katsumi Imada, Graduate School of Science, Osaka University.
P. gingivalis is known as the most important bacterium involved in periodontal disease. The bacterium belongs to the class Bacteroidia and is a major oral pathogen that is suggested to be associated not only with the onset and progression of chronic periodontitis in adults but also with other organs diseases such as rheumatoid arthritis, cardiovascular disease, and pancreatic cancer. Recently, it has been reported that P. gingivalis is also involved in the formation of β-amyloid-like deposits in the brain.
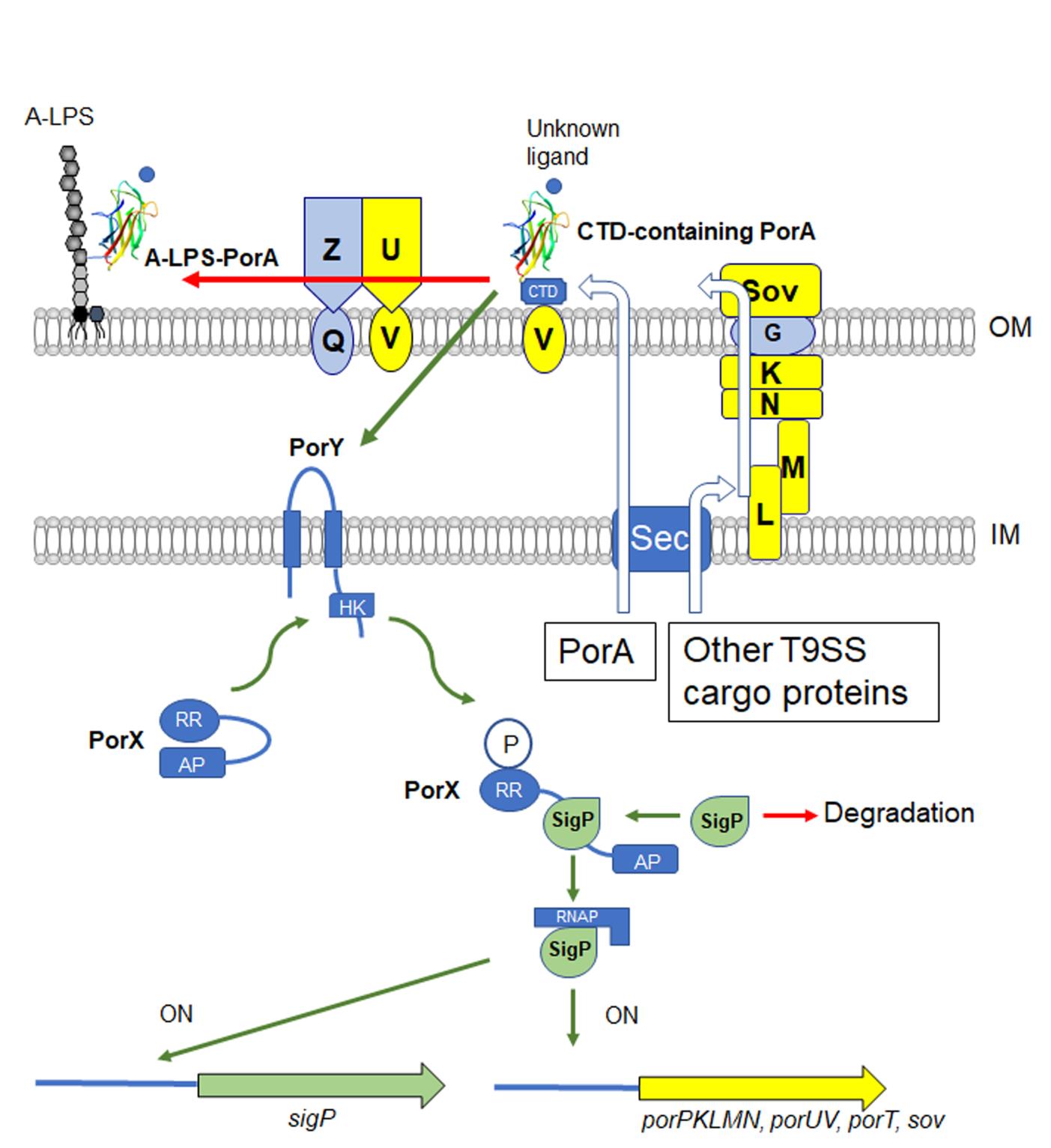
P. gingivalis secretes gingipain proteases, highly proteolytic enzymes, because the bacterium is unable to utilize carbohydrates as energy sources. A group of gingipains is considered to be the most important virulence factor of this bacterium. Gingipains and peptidylarginine deiminase, which is suggested to be associated with rheumatoid arthritis, have a consensus sequence (CTD domain) on the C-terminal side and are transported to the cell surface and outside the cell by the T9SS. There are about 30 proteins with CTD domains, some of which covalently bind to negatively charged lipopolysaccharides (called A-LPS) and attach to the cell surface. The T9SS consists of at least 15 proteins, of which PorK, PorL, PorM, PorN, PorP, PorT, PorU, PorV, Sov are regulated by the two-component regulatory system PorY-PorX and the ECF sigma factor SigP.
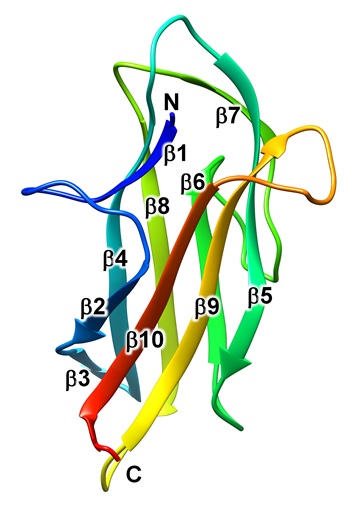
This time, we focused on PGN_0123, which is one of the CTD-containing proteins of the T9SS, and found that it is a positive regulator of the T9SS that plays a role in upstream of PorY-PorX-SigP signaling (Fig. 1). Furthermore, the structure was determined in detail by X-ray crystallography (resolution 1.3 Å) (Fig. 2). As a result, it was found that PGN_0123 is similar to the FimH protein localized at the tip of type 1 pili of Escherichia coli and having the ability to bind mannose. Based on these results, PGN_0123 protein was named PorA as a novel molecule involved in the regulation of T9SS expression. PorA has two forms, CTD-containing type and A-LPS-bound type, on the cell surface, and the former may be involved in the regulation of expression of T9SS. This discovery may lead to the development of chemicals that specifically suppress the pathogenicity of P. gingivalis.
This study has been published online in Scientific reports on Dec 3, 2020.
https://www.nature.com/articles/s41598-020-77987-y
DOI: https://doi.org/10.1038/s41598-020-77987-y
◆Contact information◆
Mikio Shoji, Ph.D.
Associate Professor
Department of Microbiology and Oral Infection
Graduate School of Biomedical Sciences
Nagasaki University
Phone: +81-95-819-7649
E-mail: m-shoji*nagasaki-u.ac.jp (change * to @)
|
Fig. 1. Proposed model of regulation of the T9SS gene expression in P. gingivalis. |
|
|
Fig. 2. Structure of the PorA protein. |