July 21, 2023
A genetically engineered Plasmodium falciparum expressing NanoLuc as an innovative tool for malaria drug discovery.
■Abstract
This study has generated a new genetically modified Plasmodium falciparum reporter line that could contribute to developing new anti-malarial drugs. This transgenic P. falciparum reporter line constantly expresses two reporter proteins, GFP and NanoLuc, at high levels in two different hosts, human and mosquito. By utilising the expression of these two reporter proteins, P. falciparum can be successfully visualised and quantified with high sensitivity. Furthermore, by using NanoLuc-derived signals, we have demonstrated the utility of the reporter line to evaluate the drug efficacy of anti-malarial compounds that are effective in multiple stages in the parasite life cycle. Using this drug platform, we identified a new compound, OU0074008, which kills the asexual blood stages of P. falciparum from a library of 1920 compounds provided by Osaka University. This study suggests that a drug discovery platform using P. falciparum expressing NanoLuc will greatly advance the development of anti-malarial drugs.
The study was carried out by a research team led by Assistant Professor Shinya Miyazaki and Research Fellow Yukiko Miyazaki (currently Assistant Professor, Department of Protozoology) and Associate Professor Daniel Ken Inaoka from Nagasaki University, Institute of Tropical Medicine, Shionogi Global Infectious Diseases Division. The research is also an international collaboration with Leiden University and TropIQ in the Netherlands, Sorbonne University in France, and Graduate School of Pharmaceutical Sciences, Osaka University. This study has been published in the journal Communications Biology (doi: 10.1038/s42003-023-05078-5, Link: https://www.nature.com/articles/s42003-023-05078-5).
■Summary
● Plasmodium falciparum infects two types of hosts, human and vector mosquitoes.
● We have created a transgenic P. falciparum that expresses two reporter proteins, GFP and NanoLuc, at high levels in both the human- and the vector mosquito stage.
● We demonstrated that the generated parasites can be used to evaluate antimalarial compounds in multi-stage.
●Our study provides a robust platform for developing anti-malarial drugs that are effective in multiple stages.
■Introduction
Malaria is a protozoan infectious disease transmitted by mosquitoes in tropical areas of Africa and South-East Asia that causes an enormous number of infections and deaths. Malaria symptoms such as fever and chills are caused by the multiplication of P. falciparum in human red blood cells (asexual blood stage). On the other hand, some portion of the parasites in the red blood cells differentiates to a stage called the gametocyte stage and are transferred into the mosquito's midgut by mosquito blood sucking. The malaria parasites that have multiplied in the mosquito host developed into a stage called the sporozoite stage, which again enters the human body through the mosquito's bite. The infected sporozoites first infect the human liver, where they undergo an incubation period before infecting human red blood cells. Thus, P. falciparum has a very sophisticated parasitic strategy for infecting two hosts, humans and mosquitoes (Fig.1).
To treat malaria, therapeutic agents have been used to kill P. falciparum in the red blood cells. However, resistance to existing anti-malarial drugs is emerging, and developing anti-malarial drugs with new modes of action is desired. P. falciparum is transmitted by mosquitoes, so drugs that prevent the spread of the parasites into the mosquito host (transmission inhibitors) are considered essential for malaria control. Furthermore, as there is an incubation period of one to two weeks in the liver after infection of humans by mosquito bites, killing the parasites in the liver can prevent malaria onset. Therefore, anti-malarial drugs that are effective in multiple stages are expected to contribute to malaria control as they can be effective as therapeutic, prophylactic or transmission inhibitors (Fig.1).
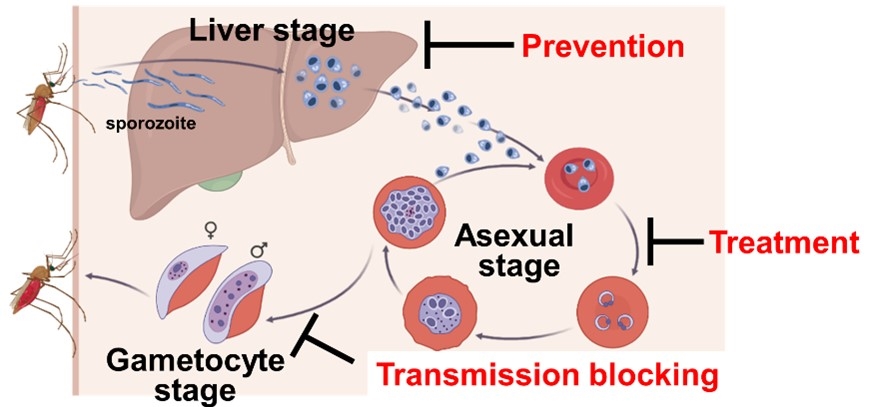 Fig.1 The whole life cycle of P. falciparum and desired anti-malarial drugs. |
■Results
In this study, we genetically modified P. falciparum to simultaneously express GFP, a fluorescent protein, and NanoLuc, a luciferase. The life cycle of P. falciparum was recapitulated in the laboratory, and the expression of GFP was confirmed in the asexual blood-, gametocyte-, oocyst-, sporozoite- and liver stages (Fig. 2). These results indicate that microscopic observation of GFP fluorescence signals facilitates the observation of Plasmodium morphology and numbers in the multiple stages.2
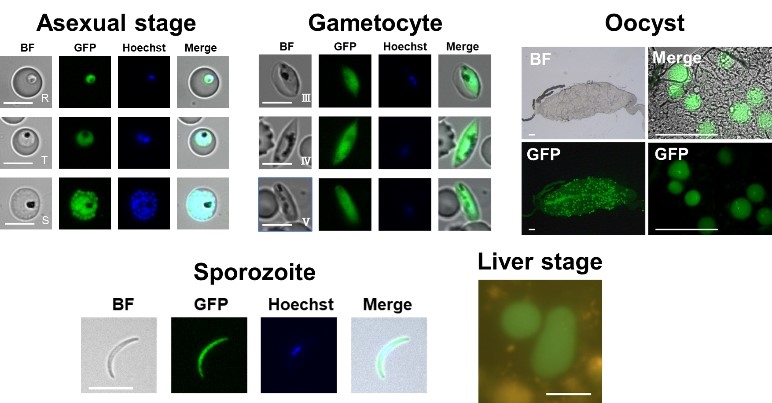 Fig.2 GFP expression in P. falciparum in the multiple stages of the life cycle. |
The NanoLuc signal, expressed simultaneously with GFP, was then used to evaluate anti-malarial drugs. P. falciparum in the asexual blood stage was seeded onto plates to which antimalarial compounds had been added, and the NanoLuc signal can be detected to determine the growth-inhibitory activity of a given compound. We found that as the concentration of the compound increased, the degree of inhibition of Plasmodium growth also increased (Fig.3). By applying this system to the gametocyte and liver stages and detecting the NanoLuc signal, we show that it is possible to evaluate anti-malarial activity not only in the asexual blood stage but also in the gametocyte and intrahepatic phases. The series of experiments suggested that it is possible to evaluate antimalarial compounds at multiple stages using the NanoLuc-expressing parasites.
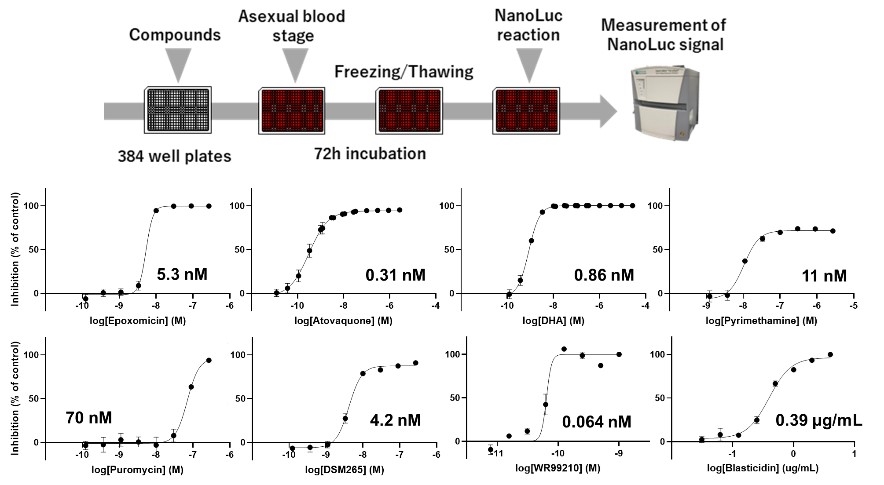 Fig.3 Evaluation of anti-malarial compounds using asexual blood stages of NanoLuc line. |
■Discussion and future perspectives
In this study, we established a new reporter, P. falciparum expressing the highly sensitive NanoLuc in both stages of humans and mosquitoes and a new drug discovery platform. By utilising the signals of GFP and NanoLuc, which are expressed in multiple stages, it is expected to greatly promote drug discovery research and basic research on P. falciparum itself. In the future, this study will be applied to the development of new anti-malarial drugs through high-throughput screening using NanoLuc-expressing P. falciparum and evaluation of the anti-malarial effects on P. falciparum in the multiple stages.
The transgenic P. falciparum reporter line developed in this study is available for transfer and collaboration with academia and drug discovery researchers upon reasonable request. If you are interested in using this reporter Plasmodium parasites, we would be happy to hear from you with the contact details at the end of this document.
▶Glossary
Malaria
Malaria infections caused by Plasmodium spp. Several species of Plasmodium are known to cause malaria in humans, this study focused on Plasmodium falciparum, which causes the most severe symptoms in humans. This parasite is transmitted from humans to mosquitoes by the blood-sucking of vector mosquitoes, multiplies in the mosquito's body and then infects other humans again by the mosquito's bite. Therefore, there is a need for a drug that is effective against both parasites in two hosts, humans and mosquitoes.
Fluorescent protein
Proteins that emit fluorescence represented by green fluorescent protein (GFP). When a fluorescent protein is exposed to light of a specific wavelength, it emits fluorescence of a different wavelength. The location and amount of fluorescent protein in the cell can be determined using particular machines to detect the fluorescence.
Luciferase
Proteins that emit luminescence through an enzymatic reaction, as represented by firefly luciferase. When luciferase reacts with a specific luminescent substance, the enzymatic reaction proceeds and light of a particular wavelength is emitted. The amount of luciferase can be measured by detecting the amount of this emitted light with a specific machine. In this study, NanoLuc, a small size and highly sensitive luciferase, was used.
High throughput screening
One of the methods of compound discovery (screening) used to find drugs with a particular effect. It is a step in which thousands to tens of thousands of compounds are evaluated using a specific assay system to identify compounds with the desired effect. In drug discovery research, it is important to find promising lead compounds at this stage and proceed to further developmental phases.
■Acknowledgement
We would like to take this opportunity to express our deepest gratitude to all those involved in this study. This paper is dedicated to the memory of our friend and colleague, Dr. Shahid Khan, who passed away on the 4th of October, 2019. This work was supported by the European Union's Horizon 2020 Research and Innovation Program under grant agreement No. 733273, and SHIONOGI & Co. Ltd. The chemical library used in this study was provided by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from AMED under grant number JP21am0101001(support number 3361).
■Papers
Journal:Communications Biology
Title:A versatile Plasmodium falciparum reporter line expressing NanoLuc enables highly sensitive multi-stage drug assays.
Authors
Yukiko Miyazaki1,2*♰, Martijn W. Vos3, Fiona J.A. Geurten2, Pierre Bigeard4, Hans Kroeze2, Shohei Yoshioka5, Mitsuhiro Arisawa5, Daniel Ken Inaoka1,6,7, Valerie Soulard4, Koen J. Dechering3, Blandine Franke-Fayard2, Shinya Miyazaki2,8*
1 Department of Molecular Infection Dynamics, Institute of Tropical Medicine (NEKKEN), Nagasaki University, 852-8523, Nagasaki, Japan.
2 Department of Parasitology, Leiden University Medical Center, 2333 ZA, Leiden, The Netherlands.
3 TropIQ Health Sciences, Transistorweg 5, 6534 AT, Nijmegen, The Netherlands.
4 Sorbonne Université, Inserm, CNRS, Centre d'Immunologie et des Maladies Infectieuses, CIMI-Paris, F-75013 Paris, France.
5 Graduate School of Pharmaceutical Sciences, Osaka University, 565-0871, Osaka, Japan.
6 School of Tropical Medicine and Global Health, Nagasaki University, Nagasaki 852-8523, Japan.
7 Department of Biomedical Chemistry, Graduate School of Medicine, The University of Tokyo, Tokyo 113-0033, Japan.
8 Department of Cellular Architecture Studies, Institute of Tropical Medicine (NEKKEN), 852-8523, Nagasaki, Nagasaki University, Japan.
♰Present address: Department of Protozoology, Institute of Tropical Medicine (NEKKEN), Nagasaki University, Nagasaki, Japan.
(doi: 10.1038/s42003-023-05078-5,
Link: https://www.nature.com/articles/s42003-023-05078-5)
*Corresponding author,
E-mail: y.miyazaki*nagasaki-u.ac.jp or smiyazaki*nagasaki-u.ac.jp
(Please change * to @.)










