March 04, 2024
In Ukr. Chem. J., an article [Part 2] of inorganic chemistry was published.
In the “Ukrainian Chemistry Journal”, an article by Daisuke Noguchi, a technical staff member of the Division of Education and Research Support, Graduate School of Engineering /, has been published. “Ukrainian Chemistry Journal” is one of the Ukrainian scientific journals.
A study on the systematic investigation into "lanthanide contraction" of the 111 lanthanide complexes coordinated with hexadentate ethylenediaminetetraacetate (EDTA), a common chelator that coordinates to metal ions with multiple atoms, has been published in the Ukrainian Chemistry Journal.
Since Ukrainian Chemistry Journal had published a research paper on EDTA and its salts (Fig. 1) by the same author in 2022 https://www.nagasaki-u.ac.jp/en/research/research77.html, the present article is the subsequent series of that.
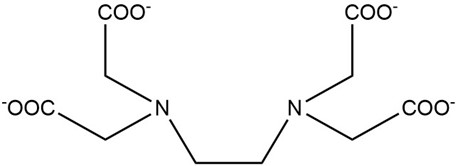 Fig. 1 Chemical structure of the tetravalent anion (EDTA−4H)4−, the conjugate base of EDTA, which is formed by deprotonation of four hydrogen atoms from ethylenediaminetetraacetic acid (EDTA). |
Herein, the lanthanides (Ln) are the metals consisting the rare earths, which include 15 elements from lanthanum (La) to lutetium (Lu). The lanthanides have applications in powerful magnets such as neodymium magnets, as well as in automotive exhaust gas catalysts and as contrast agents for nuclear magnetic resonance imaging (MRI) diagnostics.
In the lanthanides, a phenomenon called "lanthanide contraction," in which its ionic radii gradually decrease as the atomic number increases, is known. However, although more than 100 kinds of crystal structural data have been reported for EDTA-chelated lanthanide complexes, no systematic analysis has been studied.
The analysis by the present study reveals that the coordination number (CN) of the main components of the Ln-EDTA complexes is influenced by differences in the auxiliary cations, anions, and crystalline water molecules, and that there is a variety of tendency, which has been almost undiscovered in lanthanide contraction to date (Fig. 2).
The present report provides a complete set of information on lanthanide contraction of known lanthanide-EDTA complexes, and is expected to be one of the fundamental resources in advanced fields in the future.
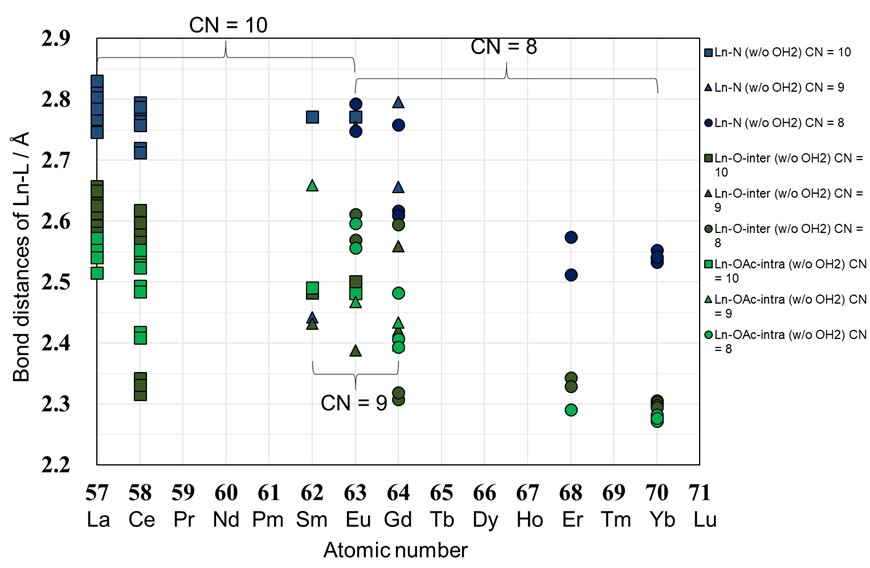 Figure 2 Average bond lengths (Å) and coordination numbers (CN) between the central lanthanide atom (Ln) and the coordinating atoms (nitrogen atom N and oxygen atom O) in the water-uncoordinated Ln-EDTA complex ion versus lanthanide atomic number (inter indicates between complex ions, intra indicates within a complex ion). |
The day when peace will be revisited to Ukraine, which is still suffering from the ravages of war, is eagerly awaited.
■Article Information
・LANTHANIDE CONTRACTION IN CHELATES OF ETHYLENEDIAMINETETRAACETIC ACID BASED ON CRYSTALLOGRAPHIC DATA: A SHORT REVIEW.
・Author:
Daisuke Noguchi (Technical Staff); Division of Education and Research Support, Graduate School of Engineering, Nagasaki University
Publication Journal: Ukrainian Chemistry Journal, Vol 89, No 9, 15-35 (2023)
URL: https://ucj.org.ua/index.php/journal/article/view/586
DOI: 10.33609/2708-129X.89.09.2023.14-34
■Acknowledgement
This study was partially supported by the Research Grant of Nagasaki University.










